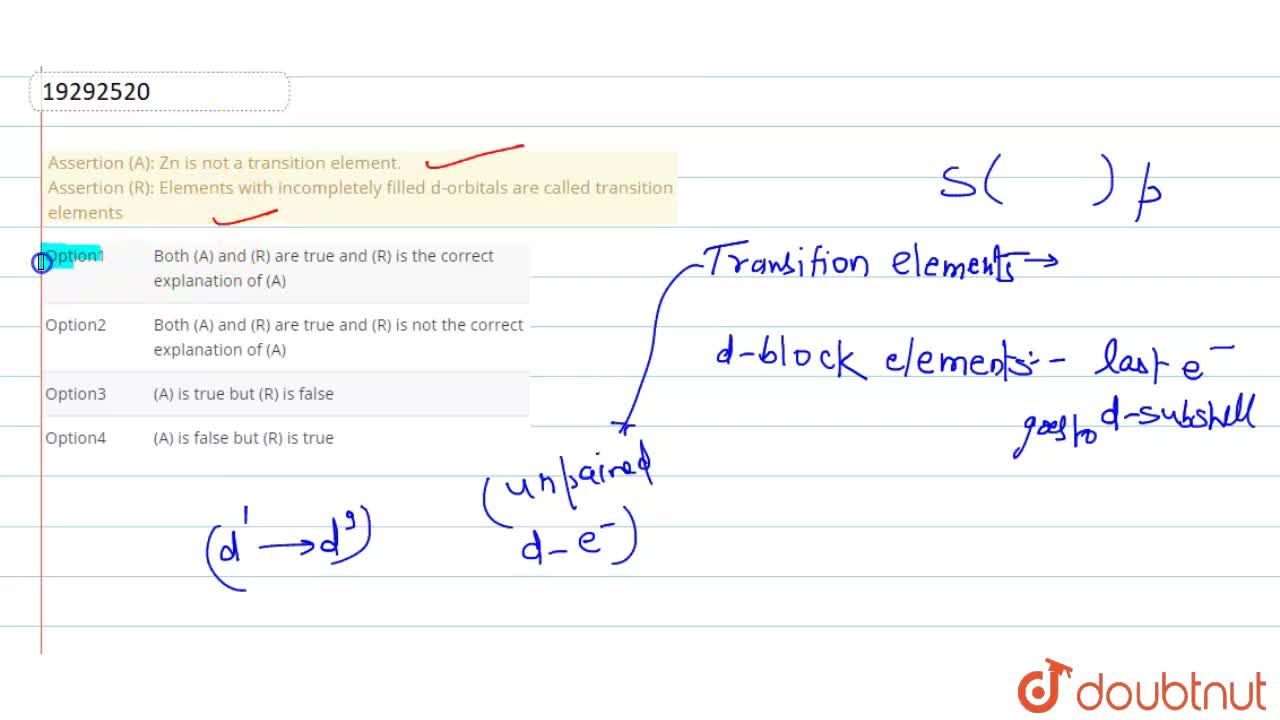
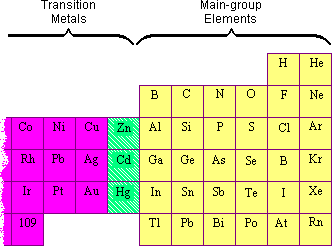
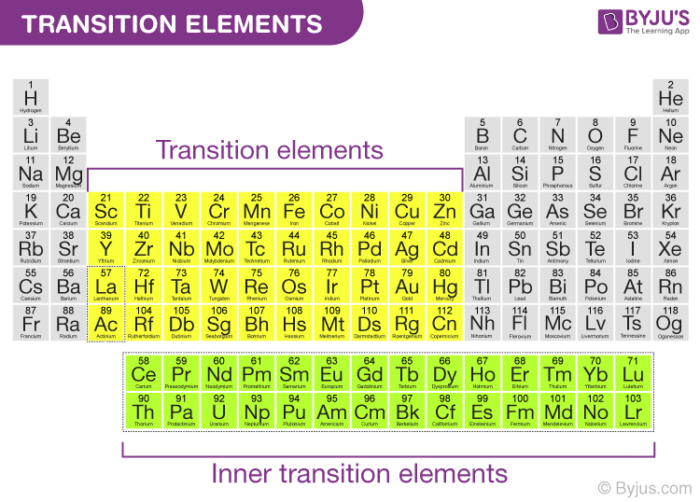
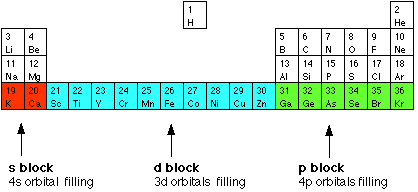
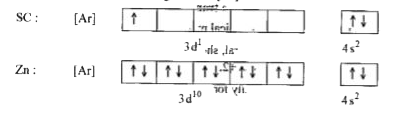
Given reasons : Zn is not regarded as a transition element. (ii) Cr^(2+) is a strong reducing agent.

On what ground can you say that scandium (Z = 21) is a transition element but zinc (Z = 30) is not? - YouTube
Why are scandium and zinc not regarded as transition metals despite their been in the transition series? - Quora

Explain the following observation: Zinc is not regarded as a transition metal - Chemistry - d- and f-Block Elements - 10669243 | Meritnation.com