DECISION OF THE EEA JOINT COMMITTEE - No 222 / 2016 - of 2 December 2016 - amending Annex I (Veterinary and p
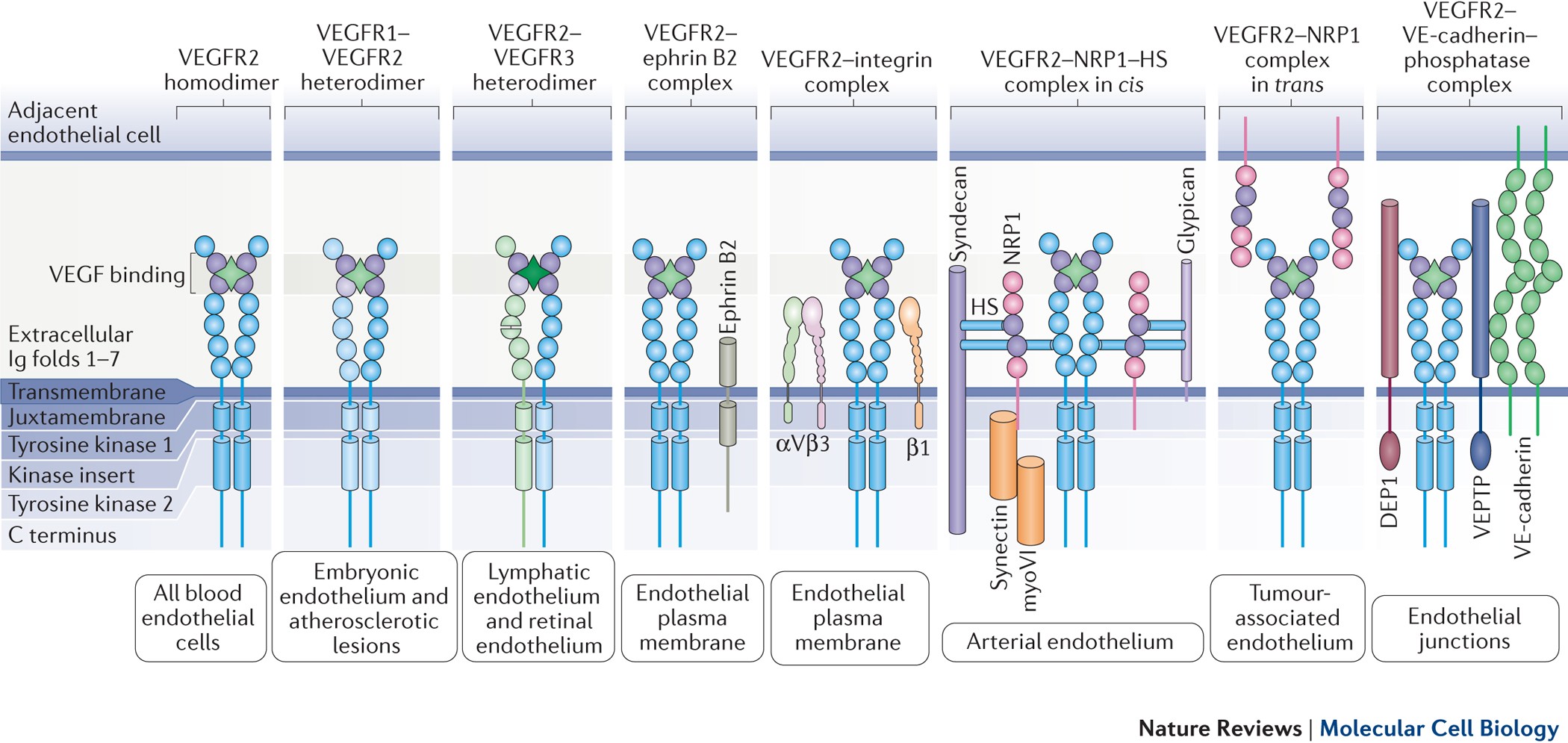
Mechanisms and regulation of endothelial VEGF receptor signalling | Nature Reviews Molecular Cell Biology

Pest categorisation of the Ralstonia solanacearum species complex - - 2019 - EFSA Journal - Wiley Online Library
Prague 28 January 2021 Information for marketing authorisation holders regarding the possibility to request issuance of a provis
EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Health systems and products Medical products – quality, saf
COUNCIL REGULATION (EU) 2019/ 999 - of 13 June 2019 - amending Regulation ( EU) No 1387 / 2013 suspending the a

Regional empowerment through decentralised governance under a centralised regulatory system facilitates the development of cellular therapy in China - The Lancet Haematology
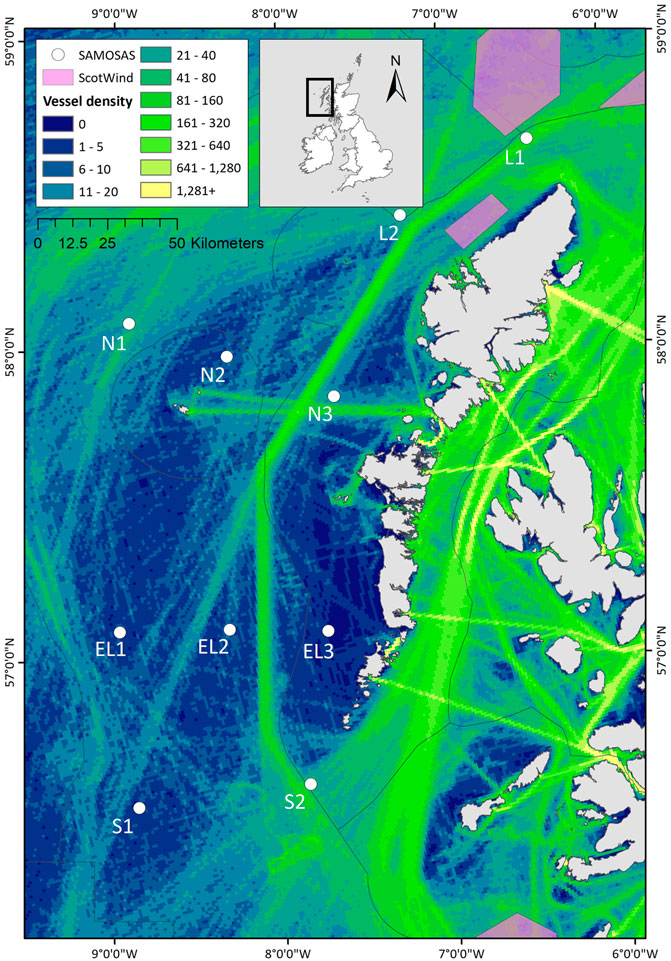
Frontiers | Monitoring cetacean occurrence and variability in ambient sound in Scottish offshore waters